505 J Application
ANDA or 505b2 Application. The guidance focuses on ANDA submissions under Section 505 j of the FDC Act petitioned ANDAs under Section 505 j 2 C and NDAs pursuant to Section 505 b 2.

Magnetic Nanoparticle Composites Synergistic Effects And Applications Mourdikoudis 2021 Advanced Science Wiley Online Library
355b2 respectively or the types of data and information that.

505 j application. 505b1 505b2 505j Application NDAANDA Pharmaceutical Concept 2021 PCThis Video is about 505b1 505b2 505j Application. NDA means New Drug Application. 355j and 21 USC.
11 What is IND approval. The 505b2 is a New Drug Application NDA mechanism which allows an applicant to seek approval for a drug product based on full safety and efficacy. About ANDA 505 j Abbreviated application that does not include safety and efficacy data.
505j Abbreviated New Drug ApplicationA route under section 505j of the FDC Act designed to allow identical or copycat drugs to be rapidly approved via an ANDA. The 505 b 1 Regulatory Pathway. Pharmaceutical Development Group Products Generic Drug Development.
Food and Drug Administration FDA drug approval pathways and represents an appealing regulatory. 314152 - Notice of withdrawal of approval of. 505 j ANDAs and Potential Diversification PDG and its staff have participated in more than 100.
Guidance Purpose and Goals Foundational guidance for applicants Assist in determining which one of the abbreviated approval. The generic version is comparable to the innovator product in terms of. What is difference between NDA and ANDA.
This pathway is used by Sponsors to obtain. In this topic we will be discussing about 505j 505b1 and 505b2 application and differences between all 3 applications. Generic Drug Application ANDA 505j An ANDA is an abbreviated submission for a drug product that is a duplicate of a previously approved drug product.
ANDA means Abbreviated New Drug. 10 What is a 505 J application. The 505 b 1 regulatory pathway is the traditional New Drug Application NDA.
Described in section 505j and 505b2 of the FDC Act 21 USC. The 505b2 new drug application NDA is one of three US. 314151 - Withdrawal of approval of an abbreviated new drug application under section 505 j 5 of the act.
A 505j application is an abbreviated new drug application ANDA that contains information to show that the proposed product is identical in active ingredient dosage. A 505 j application is an abbreviated new drug application ANDA that contains information to show that the proposed product is identical in active ingredient dosage.

Understanding Regulatory Submissions And The Role Of Regulatory Cmc Project Management
Https Www Fdli Org Wp Content Uploads 2020 10 Hegreness Matthew Pdf
Https Www Accessdata Fda Gov Drugsatfda Docs Appletter 2009 125277s000ltr Pdf
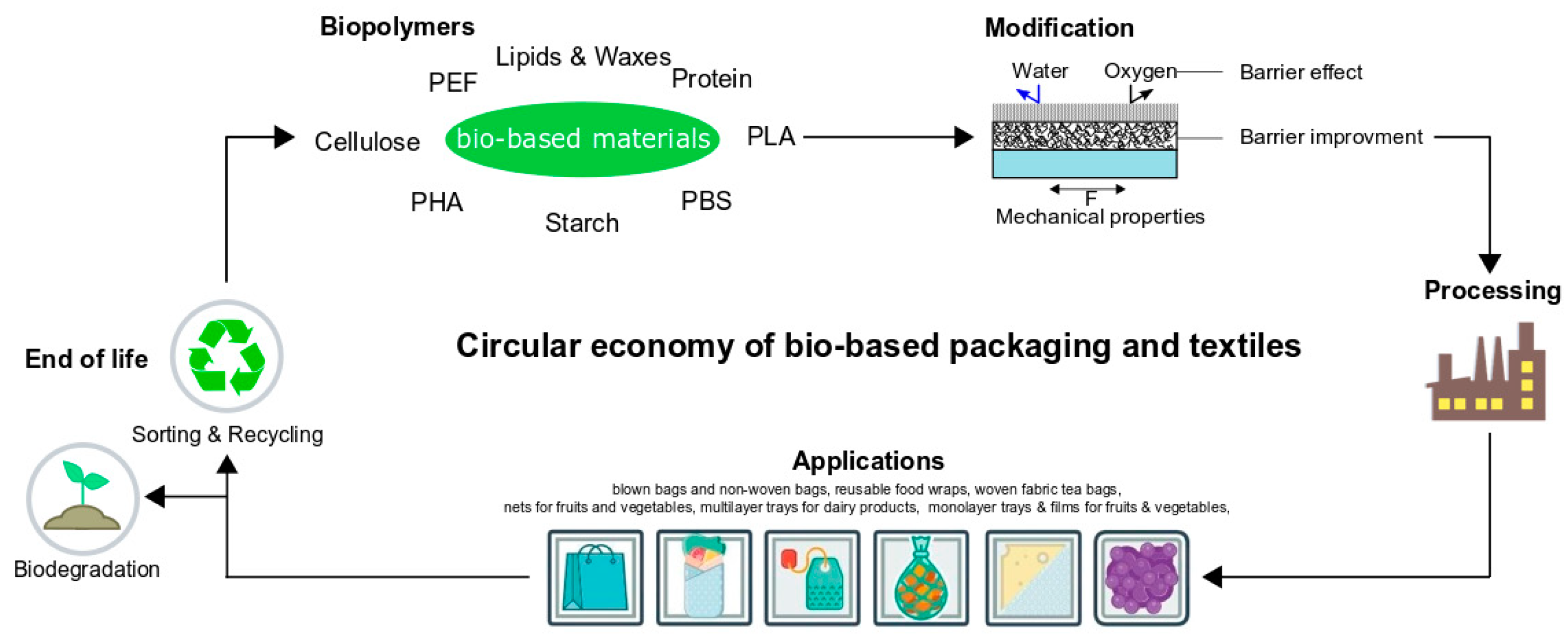
Polymers Free Full Text Bio Based Packaging Materials Modifications Industrial Applications And Sustainability Html
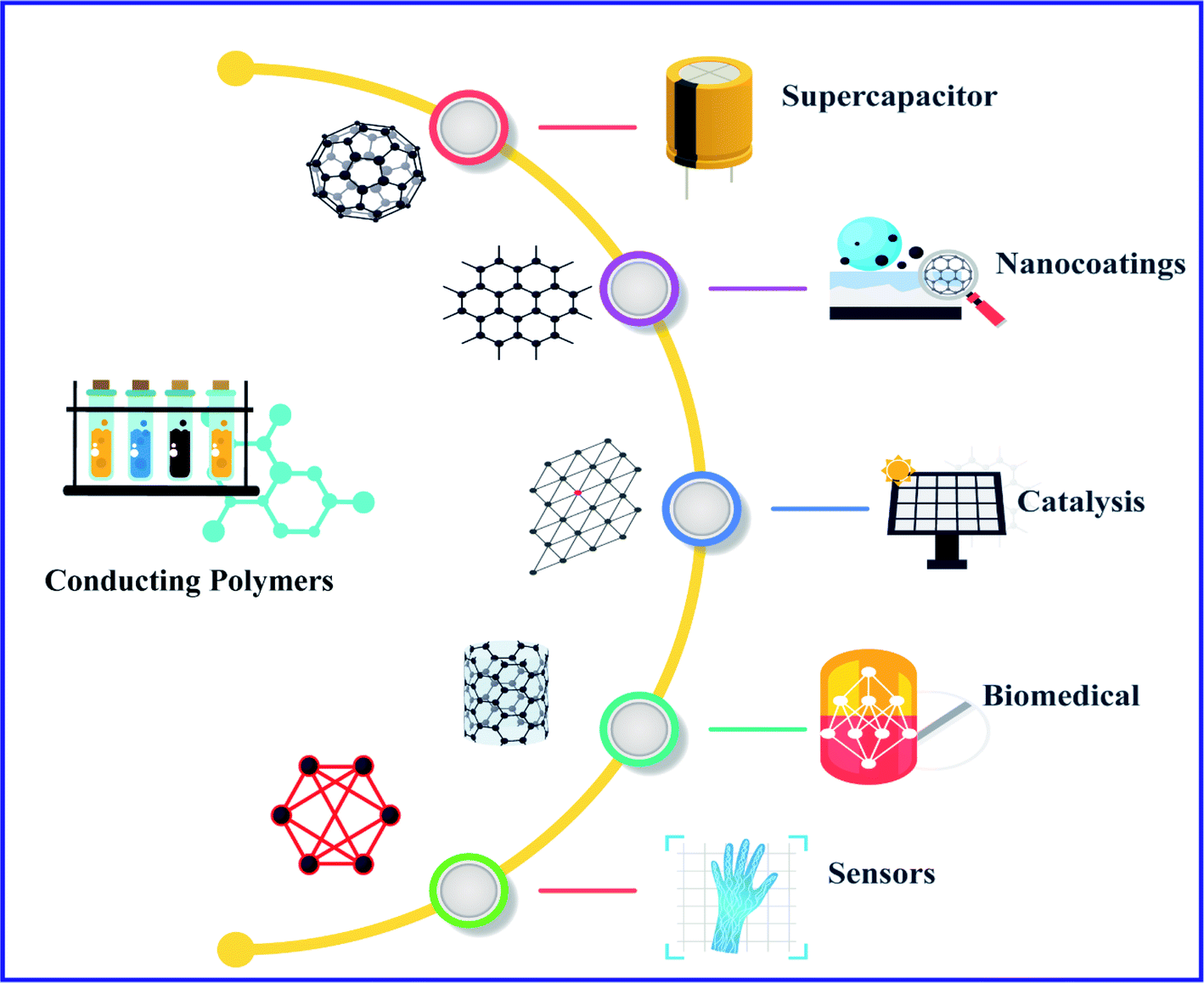
Conducting Polymers A Comprehensive Review On Recent Advances In Synthesis Properties And Applications Rsc Advances Rsc Publishing Doi 10 1039 D0ra07800j
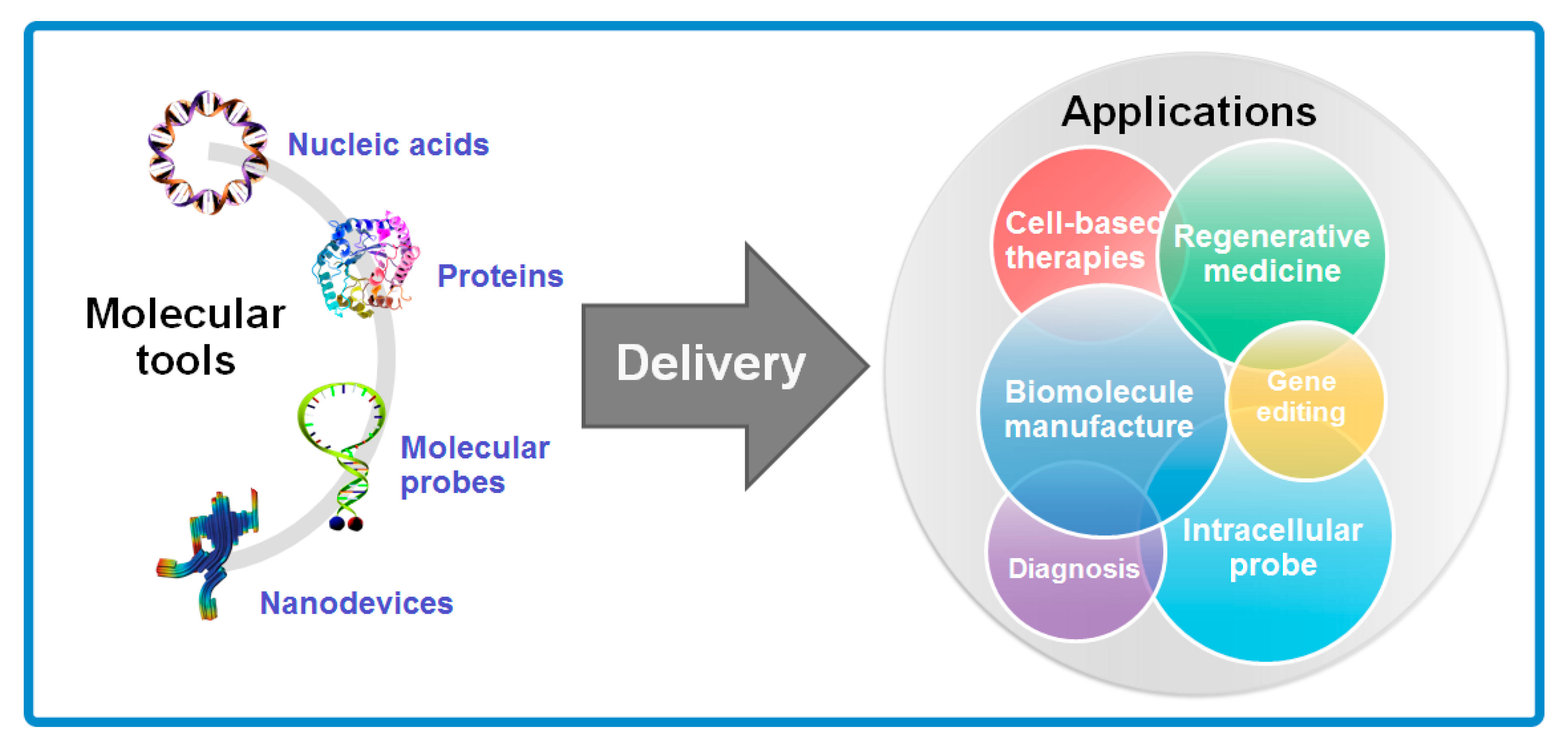
Molecules Free Full Text A Review On Electroporation Based Intracellular Delivery Html

Biosimilar Drugs Opportunities And Issues Ppt Download

Pharmaceutics Free Full Text Drug Delivery Applications Of Core Sheath Nanofibers Prepared By Coaxial Electrospinning A Review Html

The Rise Of Biosimilars Success Of The Bpcia Part I Bill Of Health

Crispr Cas Based Epigenome Editing Advances Applications And Clinical Utility Trends In Biotechnology
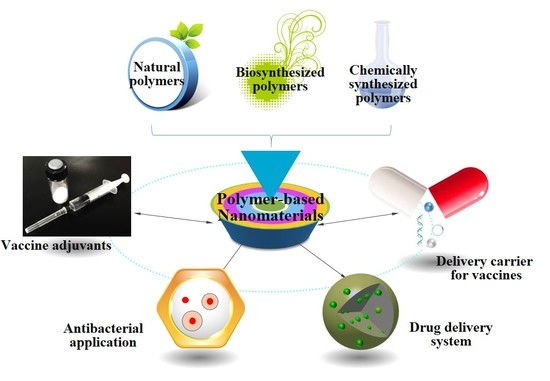
Polymers Free Full Text Polymer Based Nanomaterials And Applications For Vaccines And Drugs Html
Posting Komentar untuk "505 J Application"