5 Applications Of Grignard Reagent
R-MgBr is the Grinard regent. 5 Preparation of ketone.
Application Of Grignard Reagent Qs Study
Acts as a base taking H and produce hydrocarbons Sometimes there are the samenumber of C atoms in the Grinard.

5 applications of grignard reagent. The addition of n-butylmagnesium bromide to the following Weinreb amide furnishes 3-heptanone. How-ever its application with highly-enolizable compounds such as. Reaction of ethylmagnesium bromide with formaldehyde.
The reaction between Grignard reagents and ketones. Your Mendeley pairing has expired. Acid chloride reacts with Grignard reagent to form ketones.
Grignard Reagents are also used in the following important reactions. The magnesium species are weak electrolytes in such solvents of. The reaction of an organic halide with magnesium is not a Grignard reaction but provides a Grignard reagent.
Note that the ethyl segment joins the CH 2. Reagent and its use in the synthesis of fluconazole. A number of compounds produced by the Grignard reaction are very valuable and special intermediates or.
5 applications of grignard reagent. It has proved extremely efficient for the application to manufacture a variety of fine chemical products such as pharmaceuticals agrochemicals electronics resins and so on. Other examples of Grignard reagent.
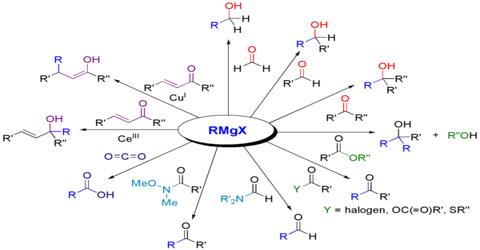
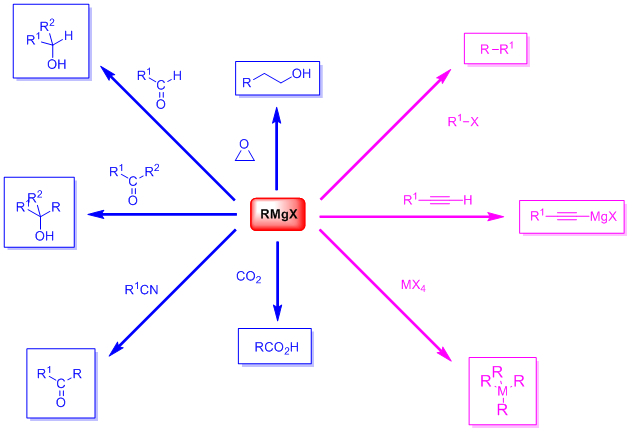
5 Grignard reactions and reagents were discovered by and are named after the French chemist François Auguste Victor Grignard University of Nancy France who published it in 1900 and was awarded the 1912 Nobel Prize in Chemistry for this work. Application of Grignard Reagent. This article reviews some of recent applications of the Grignard reaction to synthesis of alcohols ketones aldehydes acetals carboxylic acides esters ethers amino compounds organo sulfer compounds vinyl compounds acetylenic compounds and organometalic compounds.
It forms this carbanion R- like CH3CH2- This carb-anion can behave in 2 different ways. Grignard is used to extend carbon chain. Initially the Grignard reagent is added to the Weinreb amide which further undergoes hydrolysis to furnish ketone.
Dry carbon dioxide is bubbled through a solution of the Grignard reagent in ethoxyethane made as described above. Organ halogens differ significantly in their rates of reaction with magnesium. 6 Preparation of carboxylic acids.
However its application with highly-enolizable compounds such as 13-chloroacetone 3 has been previously avoided due to unfavourable side reactions. Gabriel Bolívar via ChemSketch. As shown in Scheme 6 addition of different.
Grignard reagents have been widely used on both laboratory and commercial scale and is one of the most common organometallic reagents used for the formation of carboncarbon bonds. By this reagent alkanes alcohol aldehydes ketones carboxylic acid could be prepared. Microgravimetric determination of active hydrogen by the Grignard reagent.
Electron Configuration for MagnesiumMg in Just 5 Steps To do or find or writing electronic configuration of m agnesium Mgwe will follow just 5 steps. They are frequently used for inter conversion of compoundsThis video will. To do electron configuration of m agnesium element we have to know the atomic number of the magnesium The atomic number of magnesium element is 12So magnesium has 12 electrons and 12 protons.
There is another interesting and useful property of ethereal Grignard reagent solutions. Solid carbon dioxide reacts with Grignard reagent to form addition product which on hydrolysis yields carboxylic acids. The Grignard reagent is a useful intermediate reagent in organic chemistry.
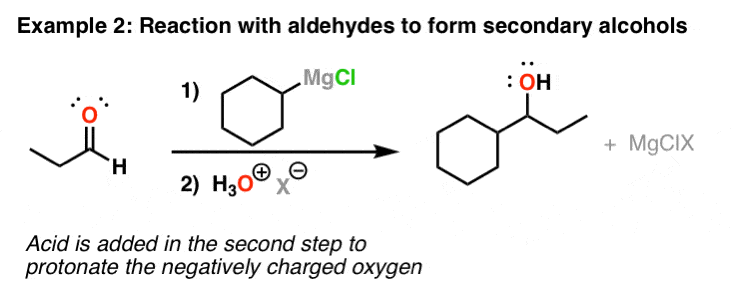
The elaboration of this intermediate was further optimized using lowcost starting. The Grignard reaction is an organometallic chemical reaction in which alkyl vinyl or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. The reaction with formaldehyde leads to a primary alcohol.
Application to analysis of impregnated paper insulating tapes. IntroductionSince the Grignard cross-coupling reaction was reported by Kumada and Tamao 1a 1b as well as Corriu and Masse in 1972 it has been used in wide range of industrial fields. Grignard reagents have been widely used on both laboratory and com-mercial scale and is one of the most common organometallic reagents used for the formation of carboncarbon bonds.
The Grignard Reaction is the addition of an organomagnesium halide Grignard reagent to a ketone or aldehyde to form a tertiary or secondary alcohol respectively. Grignard Reagents have very important synthetic applications in organic chemistry. 7 Preparation of esters.
10 The Grignard reagents are also used to prepare nitriles by reacting them with cyanogen or cyanogen chloride. Applications in synthetic chemistry it will suffice to take the easy way outsto regard and to write the Grignard reagent as RMgX. However with excess of Grignard reagent it forms tertiary alcohol.
Grignard reagents react with carbon dioxide in two stages. November 10 2020 by. The addition of an excess of a Grignard reagent to an ester or lactone gives a tertiary alcohol in which two alkyl groups.

Grignard Reaction By Vista Team123 Issuu

Grignard Reaction Simple English Wikipedia The Free Encyclopedia
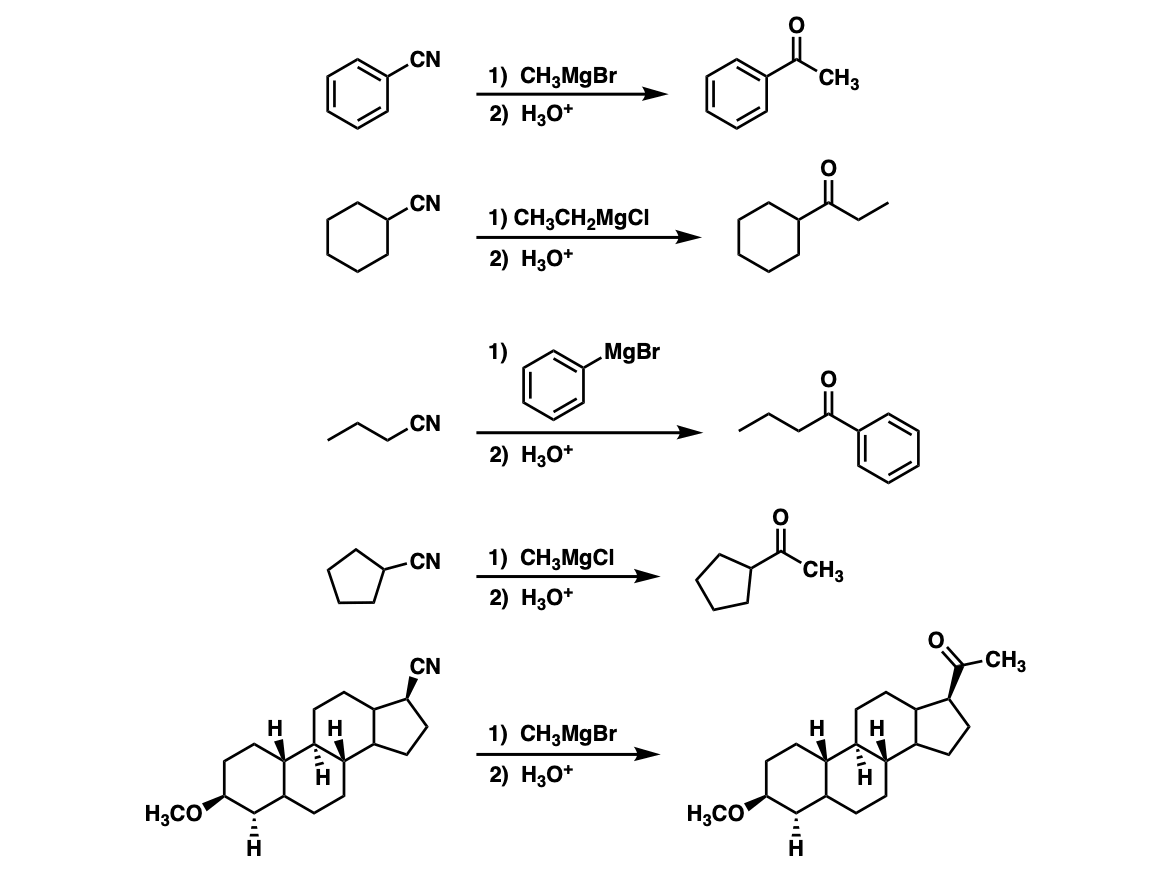
Addition Of Grignard Reagents To Nitriles To Give Ketones After Hydrolysis Master Organic Chemistry
Applications Of Grignard Reagent Online Organic Chemistry Tutor

Table 2 From Preparation Of Highly Functionalized Grignard Reagents By An Iodine Magnesium Exchange Reaction And Its Application In Solid Phase Synthesis Semantic Scholar

Grignard Reagent Reactions Ppt Video Online Download

5 Applications Of Grignard Reagent
Grignard Reagents In Organic Chemistry Master Organic Chemistry

Grignard Reagent Formation And Synthetic Applications Youtube

Posting Komentar untuk "5 Applications Of Grignard Reagent"